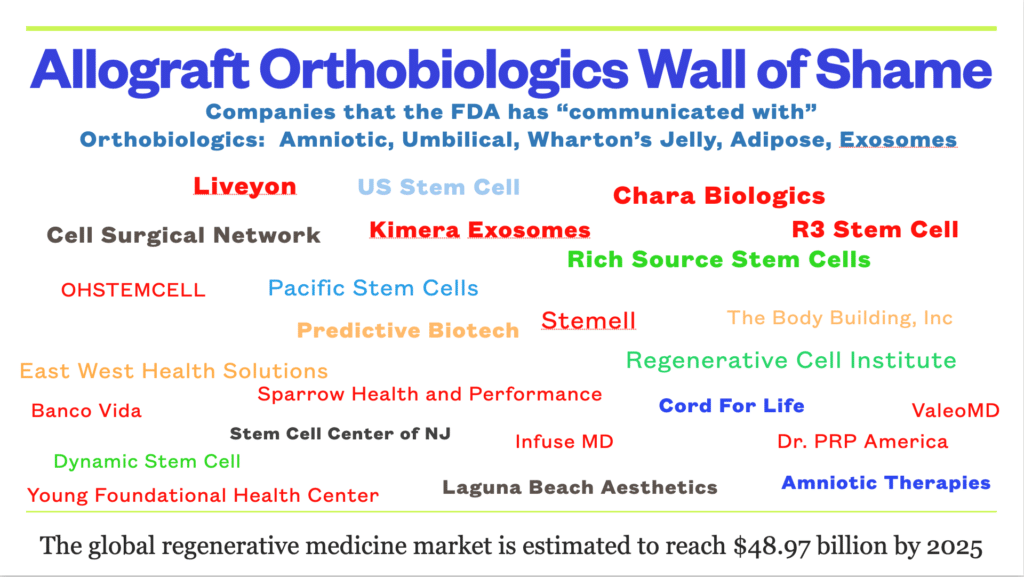
The FDA maintains a public website of their letters sent to companies in the orthobiologic field. Every company on this slide has received some form of FDA inquiry. Some of the offenses are more egregious than others with patients blinded or given tainted product resulting in septicemia and life threatening illnesses. The common factor? All used allograft orthobiologics like amniotic fluid, umbilical cord blood, Wharton’s Jelly, exosomes, or adipose (SVF); or delivered the orthobiologic in way (IV, intra-thecal, intra-ocular) or for a medical condition that had no human scientific evidence that it was safe or would work.







Post a comment
Your email address will not be published. Fields marked (*) are mandatory.