Peptides Versus PRP For Orthopedic Problems...which is better?
Why try peptides like BP-157 and TB-500 for orthopedic problems when we know that high dose PRP is better?*

Why try peptides like BP-157 and TB-500 for orthopedic problems when we know that high dose PRP is better?*

In 2024 a research group in Iran did a well designed and controlled trial comparing 5cc of placental derived exosomes with 5cc of normal saline. 29 Patients were followed for six months.....58 knees total. Patients got MRI scans before and after.
The result? There was no difference between the exosome knee and the normal saline knee in any measurable significant way.
More work to be done on this topic for sure.....at the very least so far no clinical science supports using these allograft derived exosomes in knee arthritis.

Texas Orthobiologics is your PRP (platelet rich plasma) expert and your stem cell (bone marrow concentrate) expert AND your orthopedic surgery expert all in one Dallas location! Don Buford, MD, as the clinic's founder and Director, is involved in the care of every patient directly and his 26+ years as a clinical researcher and orthopedic surgeon means he has broad experience in orthopedic regenerative medicine. As the two time President of the Interventional Orthobiologics Foundation and the recipient of the 2025 Lifetime Achievement Award from TOBI, Dr. Buford is widely recognized as one of the "go-to" experts in this rapidly growing field.
We serve local patients in Dallas, Highland Park, University Park, Preston Hollow, Mesquite, Rockwall, Heath, Flower Mound, Plano, McKinney, Allen, Southlake, Westlake, Irving, Grapevine, Fort Worth, Richardson, Farmer's Branch, Colleyville.
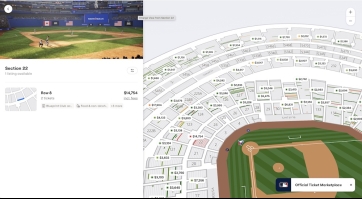
This picture shows ticket prices for an upcoming World Series game between the Dodgers and Blue Jays. For 2 people to go, it costs more than the average price for single PRP injection...even in the nosebleed bleacher seats. The game will last about 3.5 hours and other than the memories the money spent is gone that night...no ongoing daily benefits. There will be 41,500 people at this game who value this one game more than a PRP injection.
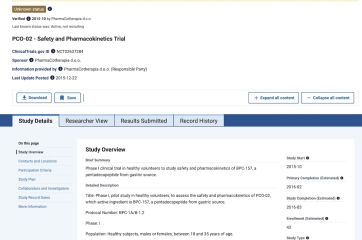
Are there any safety or placebo trials registered for the peptide BP-157?
I get this question often from clinicians and patients ask me what I mean when I say there is no human safety data.
There is no peer reviewed, publicly posted human safety data for BP–157 despite rapidly growing use spurred on by podcasts, gym talk, and social media.

Every 6 months or so I like to update my list of publications which include articles, book chapters, letters to the editor, and other references. My first co-authored paper, "The Buford Complex..." was published way back in 1994! So far in 2025, I have 4 articles/letters that have been published so far.
I stay current with the latest orthopedic and regenerative medicine research because I want my patients to have access to the best results as documented in research from around the world. Whenever I get a chance, I like to contribute and participate to this rapidly growing field within orthopedics.

*The Las Vegas Regenerative Medicine and MSK Ultrasound Course*
*January 8-10, 2026 Virgin Hotel, Las Vegas*
We are back for our 18th year!
Hotel rooms in our block are $169 with NO resort fee and this hotel is part of the Hilton Curio Collection.....earn or use your Hilton points!
Thursday is a full day of practical regenerative medicine education. We review best practices based on the best data. We cover laser and shockwave and sustained acoustic medicine. We have live demos all afternoon!
Friday is upper extremity day. Lectures before lunch and 4 hours of upper extremity hands on after lunch with1 live model for every 2 trainees and 1 faculty for every 4 trainees.
Saturday is lower extremity and spine day with the same format!

The Problem? There are no approved medical ozone devices or ozone therapies for ANY medical condition in the USA....so says the only regulatory authority....the FDA.
So, any clinic or doctor advertising ozone therapy or using an ozone device is likely using an adulterated and misbranded device and delivering an adulterated and misbranded drug. And as noted in this recent Federal lawsuit against a Richmond doctor.....patients are being harmed.
So, at this point, regardless of anyone's opinion on the medical benefits of ozone therapy.....we can't do it legally in the USA at the present time.

There is a great orthopedic meeting going on right now in Acapulco, Mexico!
This is the 29th annual AMECRA meeting which is an international meeting focused on arthroscopy and arthroplasty and reconstructive surgery.
The thought leaders in orthopedics and regenerative medicine in Mexico are here and I am looking forward to learning their approaches to helping patients with orthopedic challenges.

Can a stem cell (bone marrow concentrate) injection outperform a PRP injection in the small joints of the hand/wrist?
It's a question I often get asked.....with such a small volume injection is there still a potential benefit of doing BMC over PRP?
Well, this surgeon had PRP before we met 3 years ago and he did feel PRP worked better and lasted longer than steroid. Then we met and he wanted to try BMC. Fast forward 3 years and he has done significantly better ...about 3x longer benefit.... with BMC versus PRP.
I get that this is a case study and a patient testimonial...but it is still true and something to consider when advising patients about the potential outcomes of out orthobiologic options!