 Creska? An off-the shelf adipose product with living cells registered under FDA section 361? Many Questions pop to mind...
Creska? An off-the shelf adipose product with living cells registered under FDA section 361? Many Questions pop to mind...
Q> Have I seen a regenerative medicine company claim that I should use their product in orthopedics and that their product has living cells before? Despite being frozen, shipped and then immediately thawed in a my office?
A> Yes... And subsequent independent publications proved those claims of living cells to be 100% false
CLICK HERE FOR LINK TO PAPER SHOWING NO LIVING STEM CELLS IN ALLOGRAFT PRODUCTS
Additionally, the FDA sent out more Warning and Untitled Letters to companies marketing those products to take them off the market than I can remember to count (plus what happened in 2021....)
Now we have "Creska™"
The company claims:
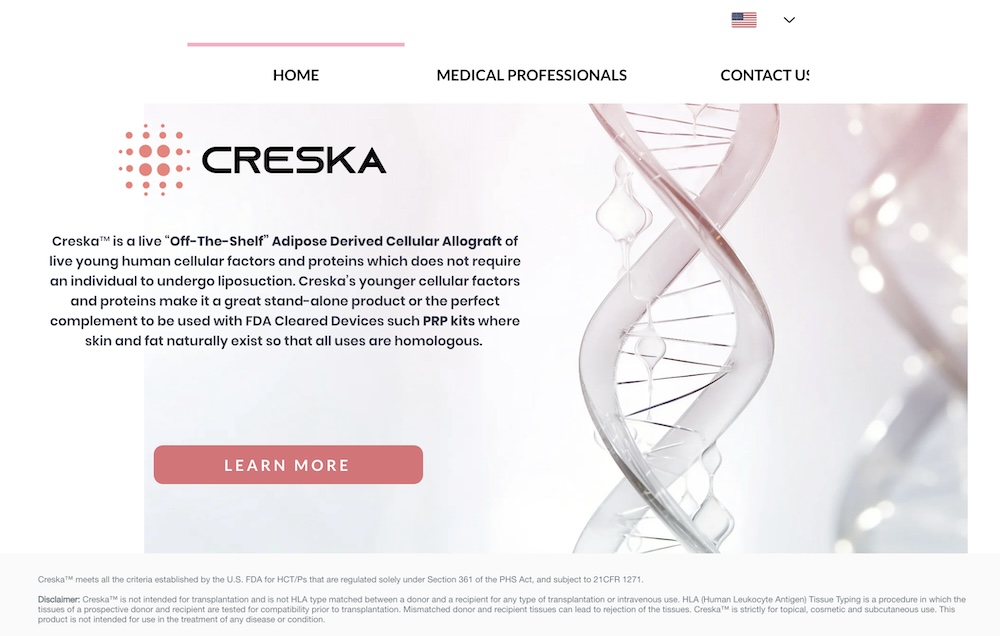
#1 "Creska is an FDA Compliant Live Cellular Adipose Derived Allograft that can be used subcutaneously, in the dermis or topically where skin or fat naturally exists to act homologously..."
#2 "Creska™ meets all the criteria established by the U.S. FDA for HCT/Ps that are regulated solely under Section 361 of the PHS Act, and subject to 21CFR 1271."
#3 "Creska™ is not intended for transplantation and is not HLA type matched between a donor and a recipient for any type of transplantation or intravenous use. .. Mismatched donor and recipient tissues can lead to rejection of the tissues. Creska™ is strictly for topical, cosmetic and subcutaneous use. This product is not intended for use in the treatment of any disease or condition.
The company clearly has no "on-label" FDA approved indication and they have registered the product under the free, "scout's honor", 30 minute, on-line FDA section 361 pathway. So, how could I use this in orthopedics? I don't know.
The company's claim of having a living cellular product would seem to automatically make their product regulated under section 351. I say that only because I have heard FDA officials say that in public forums and webinars over the past 5-8 years.....especially when amniotic fluid companies and Wharton's jelly companies and Placental tissues companies used to claim they had living cells in their products. The FDA has stopped companies from selling cosmetics with claims of living stem cells before!
Why would Creska not also be a misbranded and misregulated section 361 product claiming live cells?
I am not an attorney and I don't work for the FDA.....so ask the your health care regulatory attorney and the FDA for their opinion on this new product claiming to have living cells in an off the shelf allograft adipose product.
By their own web site.....it shouldn't be used any deeper than subcutaneously anyway 😉
And all of this before we even have safety data (and obviously no outcomes data!!!) on human orthopedic uses....so there is no way I could use this product over PRP....and to use it with PRP would likely make my PRP run afoul of the FDA too!! DYOR.







Post a comment
Your email address will not be published. Fields marked (*) are mandatory.